Risklick Revolutionizes Clinical Trials with AI-Driven Protocol AI
The landscape of clinical trials for medical devices has taken a transformative leap forward. Risklick, a Swiss startup and spin-off from the University of Bern, has unveiled its groundbreaking product, Protocol AI. This innovative software, driven by artificial intelligence (AI), is set to redefine the clinical trial process for medical devices, streamlining development and enhancing patient access to life-saving treatments. With a focus on efficiency, cost-effectiveness, and quality, Protocol AI promises to address some of the most pressing challenges in the medical device industry.
Protocol AI: The Challenges of Clinical Trials for Medical Devices
Bringing a medical device to market is a long and complex journey, typically spanning three to seven years. Within this timeline, the clinical investigation phase is both the most time-intensive and the costliest. Every clinical trial begins with the creation of a protocol, which serves as the foundation for the study’s design and execution. However, developing a protocol often takes six months of rigorous effort. Even small mistakes in the protocol can result in delays, increased costs, or failed trials.
Adding to this complexity, the medical device industry faces unique challenges compared to the pharmaceutical sector. Unlike pharmaceuticals, the medical device field is less standardized and has limited clinical trial experience. Regulatory demands are also becoming more stringent, further complicating protocol development. Consequently, these factors impact the availability, cost, and success of new medical devices, delaying patient access to innovative solutions.
Protocol AI: A Game-Changer in Clinical Trial Development
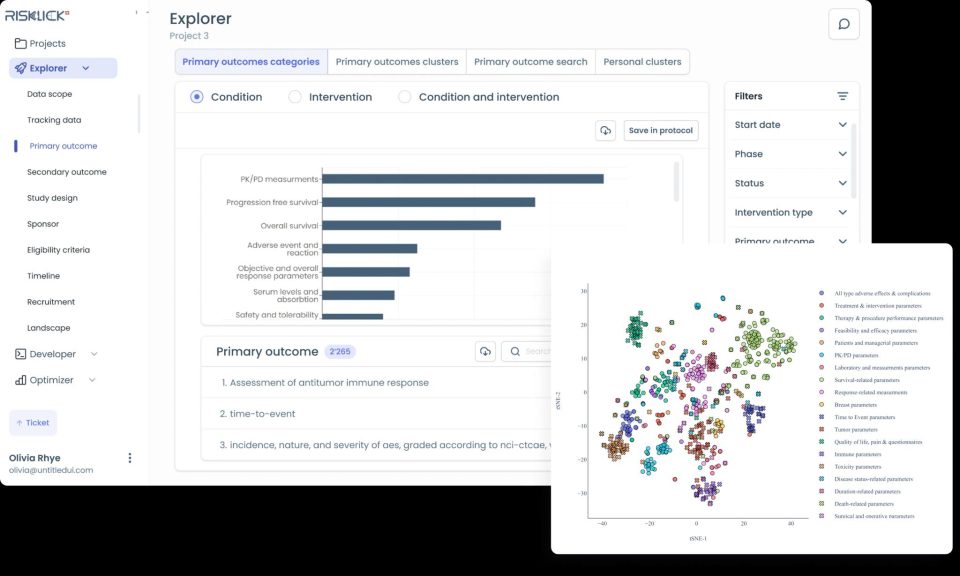
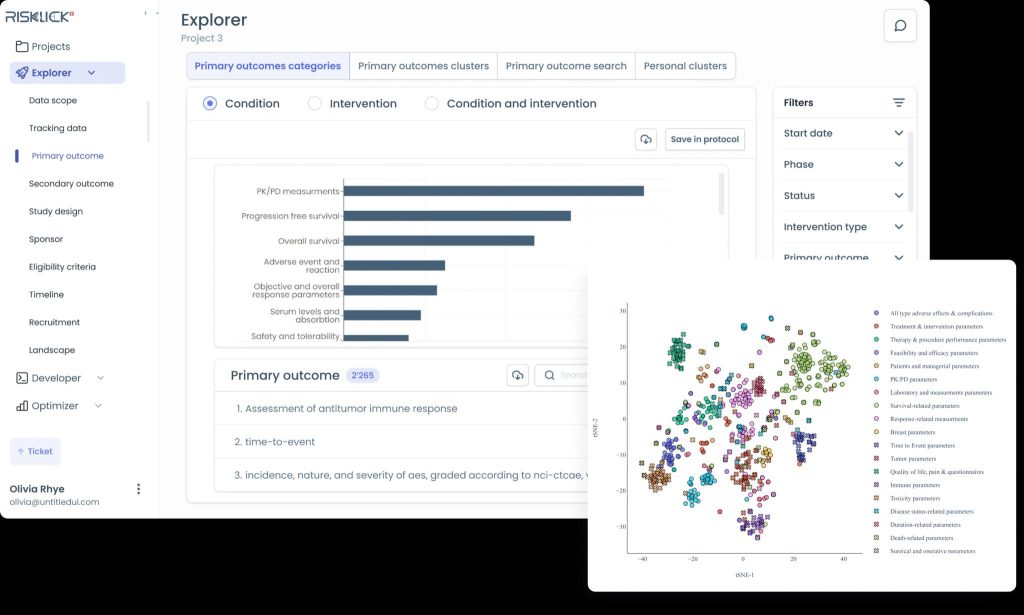
In response to these challenges, Risklick has introduced Protocol AI, a first-of-its-kind software designed specifically for medical device clinical trials. This cutting-edge solution leverages advanced Natural Language Processing (NLP) and Machine Learning (ML) technologies to optimize the development of clinical trial protocols. By analyzing existing clinical data, regulatory documents, and published studies, Protocol AI provides evidence-based insights that streamline the protocol development process.
Poorya Amini, founder and CEO of Risklick, highlights the software’s transformative potential:
“Our pioneering solution reduces both the time and cost of clinical trials, enabling faster patient access to new treatments. With Protocol AI, we have already achieved a 50% reduction in study document development time for medicinal products. We expect similar results for medical devices, improving millions of lives worldwide.”
Accelerating Innovation with AI-Powered Efficiency
Protocol AI is designed to automatically draft clinical trial protocols within minutes, significantly reducing the time required for manual development. The software integrates cutting-edge Large Language Models (LLM) to deliver high-quality, evidence-based protocol drafts. This not only accelerates the development process but also increases the likelihood of trial success by ensuring protocol accuracy and compliance with regulatory standards.
Moreover, Protocol AI empowers clinical experts to focus on strategic decisions rather than administrative tasks. By automating the protocol drafting process, the software allows researchers to allocate their time and expertise toward more critical aspects of clinical trial design. This streamlined approach ultimately accelerates innovation, bringing new medical devices to market faster and more efficiently.
Enhancing Patient Access to Life-Saving Treatments
One of the most significant benefits of Protocol AI is its potential to improve patient access to life-saving medical devices. By reducing the time and cost associated with clinical trials, the software enables manufacturers to introduce innovative devices to the market more quickly. Patients, in turn, gain faster access to advanced treatments that can improve their quality of life.
Additionally, Protocol AI’s cost-reduction capabilities may lead to more affordable medical devices. Lower development costs translate to reduced financial barriers for patients, making cutting-edge treatments more accessible to a broader population. This aligns with Risklick’s mission to enhance global healthcare outcomes through innovative technology.
A Collaborative Effort with Industry Leaders
Risklick’s success with Protocol AI has already attracted notable partners from the pharmaceutical and medical device industries. Collaborations with organizations such as Debiopharm, a leading biotechnology company, and ISS AG, a Swiss clinical research company specializing in medical devices and digital medicine, underscore the software’s credibility and potential. These partnerships highlight the growing recognition of Protocol AI as a game-changing solution in clinical trial development.
Revolutionizing the Medical Device Industry
Protocol AI represents a significant milestone in the evolution of clinical trials for medical devices. By addressing the unique challenges of protocol development, the software sets a new standard for efficiency, quality, and patient-centricity. Its ability to reduce protocol development time by 50% not only accelerates innovation but also paves the way for improved patient outcomes worldwide.
This technological breakthrough comes at a time when the medical device industry is under increasing pressure to meet rising regulatory demands while maintaining cost-effectiveness. Protocol AI’s ability to navigate these challenges positions it as an indispensable tool for clinical researchers, manufacturers, and healthcare providers alike.
A Vision for the Future
Risklick’s vision extends beyond streamlining clinical trials. By leveraging AI-driven solutions like Protocol AI, the company aims to foster a more efficient and patient-focused healthcare ecosystem. The software’s success in reducing development timelines and costs underscores the transformative potential of AI in addressing global healthcare challenges.
As the medical device industry continues to evolve, innovative solutions like Protocol AI will play a crucial role in shaping its future. By empowering researchers with advanced tools and enabling faster patient access to treatments, Risklick is driving meaningful change in the way clinical trials are conducted.
Conclusion
Risklick’s Protocol AI is more than just a technological innovation. It is, in fact, a catalyst for change in the medical device industry. It has an ability to streamline protocol development, reduce costs, and improve patient access to life-saving treatments. Therefore, Protocol AI is poised to revolutionize clinical trials on a global scale. Risklick continues to collaborate with industry leaders and push the boundaries of innovation. Thus ensuring future of medical device development looks brighter than ever.
Key CX Takeaways from Protocol AI’s Launch
- Faster Patient Access to Medical Devices
- Impact: Protocol AI drastically reduces clinical trial development timelines, cutting protocol drafting time by 50%. This accelerated timeline allows patients to access innovative and life-saving medical devices faster, improving overall patient satisfaction.
- Customer Experience (CX) Focus: Patients benefit from quicker solutions to health challenges, reinforcing trust in healthcare providers and device manufacturers.
- Cost-Effective Innovation for Manufacturers
- Impact: By streamlining the clinical trial process, Protocol AI reduces costs associated with protocol development and regulatory compliance. This financial relief can translate into more affordable medical devices for end-users.
- CX Focus: Lower costs can reduce the financial burden on patients, creating a more accessible and equitable healthcare ecosystem.
- Enhanced Protocol Accuracy and Quality
- Impact: Protocol AI uses AI-driven insights, including Natural Language Processing (NLP) and Machine Learning (ML), to create precise and evidence-based protocols. This minimizes errors and increases the likelihood of clinical trial success.
- CX Focus: Improved accuracy ensures that patients receive safe, effective treatments, boosting confidence in medical devices and the companies behind them.
- Streamlined Decision-Making for Researchers
- Impact: The automation of protocol drafting frees up clinical experts to focus on strategic decision-making. This optimizes resource allocation and allows for faster progression through clinical trials.
- CX Focus: Faster approvals and device launches enhance patients’ experience by addressing unmet healthcare needs more promptly.
- Improved Global Healthcare Accessibility
- Impact: Protocol AI has the potential to benefit millions worldwide by making medical devices more accessible. Its efficiency aligns with global efforts to reduce healthcare disparities.
- CX Focus: Patients in underserved regions could access advanced medical technologies faster, specifically, ensuring broader healthcare inclusion.
- Transparency in AI-Driven Innovation
- Impact: Protocol AI, in fact, fosters transparency in clinical trial design by using evidence-based insights regulatory-aligned methodologies.
- CX Focus: Patients and stakeholders can trust the reliability of clinical trials, particularly, fostering loyalty to healthcare brands utilizing AI solutions.
- Collaboration with Leading Industry Players
- Impact: Partnerships with major companies like Debiopharm and ISS AG validate not only Protocol AI’s credibility but effectiveness as well.
- CX Focus: In fact, such collaborations assure patients of the technology’s reliability, and, instill confidence in the medical devices launched using this innovation.
- Scalability and Flexibility Across Trials
- Impact: Protocol AI can adapt to various clinical trial needs across medical devices, in fact, from small-scale to large-scale trials.
- CX Focus: Scalability ensures a consistent patient experience, as a matter of fact, irrespective of the size or complexity of the trial.
Speed, Accuracy, Cost Reduction, and Inclusivity with Protocol AI
Speed, accuracy, cost reduction, and inclusivity, are the key differentiating factors. By focusing on these, in fact, Protocol AI significantly elevates the customer experience for patients, researchers, and device manufacturers alike. Moreover, setting a new benchmark in healthcare innovation.